The Self-Lab Assessment Tool (S-LAT) is a do-it-yourself checklist and planning workbook that helps laboratory teams evaluate their biorisk management—then auto-build a corrective action plan. It was adapted by the Elizabeth R. Griffin Program to suit real lab conditions, especially in places with infrastructure constraints, and can be completed internally by lab staff (no outside auditors required). (Elizabeth R. Griffin Program)
S-LAT covers six practical areas: infrastructure & equipment, administration & training, biorisk management system, specimens (inventory, collection, handling, referral/transport), and waste management—plus an Action Plan sheet that fills itself as you log corrective actions. (Elizabeth R. Griffin Program)
It’s available free (English/French; guide + Excel tool) and has been field-tested in multiple countries, including pilots in Morocco and Libya. (Elizabeth R. Griffin Program, biosecuritycentral.org)
Why it matters: S-LAT is aligned with the modern, risk- and evidence-based approach promoted in WHO’s Laboratory Biosafety Manual, 4th ed. (LBM4) and complements other frameworks like BMBL. It helps you turn everyday practice into measurable safety improvements. (World Health Organization, CDC)
Who should run it (and what to prepare)
Ideal leads: Biosafety Officer/Lab Manager with support from section heads (bacteriology, virology, etc.), facilities, and procurement/finance.
Before Day 1, set up:
- Download the S-LAT Guide and the Excel workbook. (Elizabeth R. Griffin Program)
- Create a shared folder for proof (SOPs, training logs, maintenance records, photos of labels/tags).
- Decide what rooms/units to include first (e.g., BSL-2 bacteriology, sample reception, cold room).
- Block 1–2 hours/day for the core team.
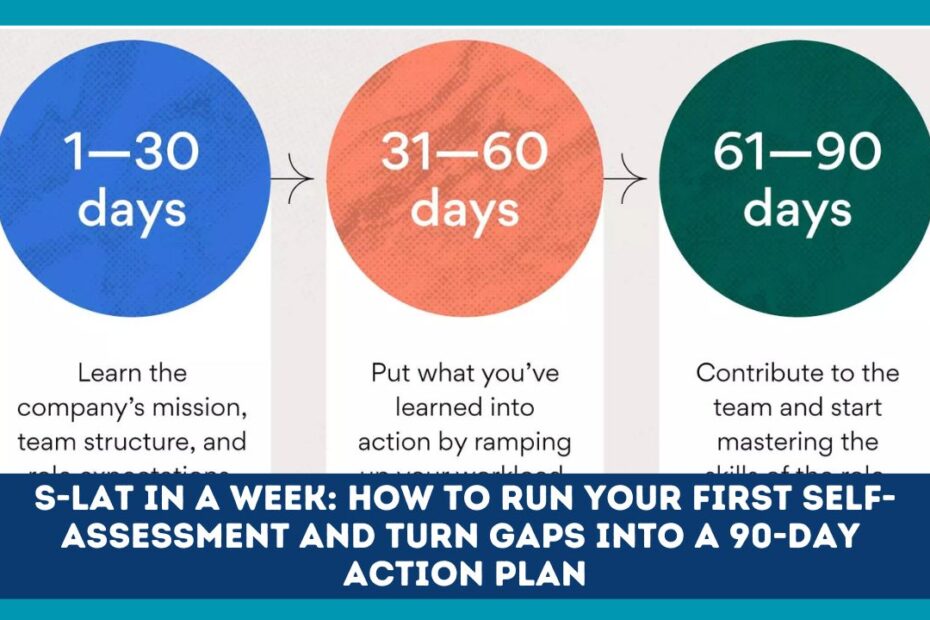
Your 5-Day Sprint Plan
Day 1 — Kick-off & Scope (90–120 min)
- Walk through what S-LAT checks and agree what’s in scope this week.
- Assign module owners and show how to record “Response” + short comment + corrective action in the Excel sheet; this is critical because those actions auto-populate the Action Plan tab. (Elizabeth R. Griffin Program)
- Make a proof list (what photos/docs you’ll capture for each gap).
Outcome: Everyone knows their section; the team understands how the action plan is generated.
Day 2 — Infrastructure & Equipment (walk-through + desk check
Open the Infrastructure tab and review: building condition, ICT, utilities, equipment inventory, service/monitoring/calibration. Photograph eyewash tags, autoclave logs, BSC certificates, breaker labels, and any missing labels/signage. (Elizabeth R. Griffin Program)
Common gaps you might log today
- No eyewash inspection tags in certain rooms.
- Autoclave cycle records not filed monthly.
- UPS or generator not tested/recorded for key freezers.
Enter each as a short corrective action (who/what/where).
Day 3 — Administration & Training (records review)
Open Admin_Training. Check org docs, personnel qualifications, quality management, SOP currency and training (topics, frequency, competency sign-offs, fit testing, vaccination policy where applicable). (Elizabeth R. Griffin Program)
Typical fixes
- Add annual refresher for spill response and ocular exposure.
- Update biosafety manual to reference LBM4 risk-based terms (GMPP, incident reporting, etc.). (World Health Organization)
Day 4 — Biorisk System + Specimens (observe the workflow)
Open BRM_System and Specimens. Check policies/docs, risk assessment, mitigation (admin + practice), PPE, security, emergency procedures, performance review; then follow a sample from receipt → processing → storage → referral/transport. Note labeling, secondary containment, courier hand-off, chain-of-custody details. (Elizabeth R. Griffin Program)
Useful proof
- Photos of secondary containers with absorbent material.
- A completed referral form and transport SOP reference.
Day 5 — Waste & Action Plan (prioritize and schedule)
Open Waste_Mgt (disinfection, segregation & storage, policies/SOPs). Then go to Action Plan—your earlier “corrective actions” will appear automatically here. Assign priority (Low/Med/High), due date, and one owner for each item. Export as PDF for leadership sign-off. (Elizabeth R. Griffin Program)
Tip: Keep the first plan small and shippable—aim for 10–15 actions you can close within 90 days.
A Ready-to-Use 90-Day Action Plan Template
| Priority | Gap (from S-LAT) | Corrective Action | Owner | Due | Evidence to attach |
|---|---|---|---|---|---|
| High (30 d) | No eyewash tags in BSL-2 | Service all eyewashes; tag & log monthly | Facilities lead | 30 days | Photo of tags + log extract |
| High (45 d) | No spill refresher in past 12 mo | Schedule 2 sessions; record attendance & competency | BSO | 45 days | Roster + quiz results |
| Med (60 d) | Incomplete autoclave records | Standardize log; post near unit; weekly review | Section head | 60 days | New log + signed weekly checks |
| Med (90 d) | Specimen referral gaps | Update SOP + quick-guide; brief couriers | QA manager | 90 days | SOP vX.Y + briefing sign-in |
| Low (90 d) | Old biosafety manual | Align with LBM4/BMBL terms and practices | BSO | 90 days | Change log + approval note |
Why align with LBM4/BMBL? These references anchor your fixes in globally recognized risk-based practice and safety culture, which auditors and partners expect. (World Health Organization, CDC)
How to score quick wins vs. bigger lifts
- Quick wins (≤30 days): labels, logs, missing signs, short refreshers, re-posting SOPs.
- Medium (31–90 days): training cycles, waste vendor contracts, PPE fit-testing catch-up.
- Longer (>90 days): equipment replacement, room modifications, system-level policy changes.
What “good” looks like after 90 days
Track a few metrics and show the trend:
- % of actions closed on time (target ≥80%).
- Time to first response for incidents/near-misses (hours).
- Training completion + competency for the at-risk tasks you flagged.
- Repeat-incident rate for the same step/equipment (should drop).
These indicators reflect the learning culture emphasized in LBM4. (World Health Organization)
Common pitfalls (and easy fixes)
- Action bloat: If the plan has 40 items, split it into quarterly sprints.
- No single owner: Every action needs one accountable person.
- Proof not saved: If it isn’t documented, it didn’t happen—attach photos/logs.
- Over-prescription: Keep controls proportionate to risk, per LBM4. (World Health Organization)
Download & Use (free)
Grab the official S-LAT Guide (PDF) and the Excel tool (English/French). The Excel file auto-generates your Action Plan as you type corrective actions—so fill responses electronically. (Elizabeth R. Griffin Program)
If you need a quick overview and background (languages, authors, pilots), see the Biosecurity Central entry; it also notes availability in English, French, and Arabic. (biosecuritycentral.org)
FAQs
1) How is S-LAT different from a full external audit?
S-LAT is a self-assessment designed for facility-level teams. You can finish a focused scope in a week and walk away with a prioritized 90-day plan. For broader, deeper reviews later, pair S-LAT with tools like WHO’s Laboratory Assessment Tool or national frameworks. (Elizabeth R. Griffin Program)
2) Do we need to answer every question?
No. The guide explicitly says to select the components/questions appropriate for your lab and to generate actions only where there’s a gap. The goal is practical improvement, not ticking every box. (Elizabeth R. Griffin Program)
3) Can we align this with global guidance?
Yes. S-LAT’s structure and your action plan can reference LBM4 (risk-based, evidence-based safety culture) and BMBL (protocol-driven risk assessments), which strengthens your documentation and training updates. (World Health Organization, CDC)
Closing note
If you invest one focused week and keep your first 90-day plan tight, S-LAT will give you a clean baseline, visible improvements, and a repeatable rhythm you can re-run every quarter. It’s a simple way to prove progress—to your team, leadership, and partners—while making day-to-day lab work safer and smoother. (Elizabeth R. Griffin Program)